The Interactions Of Earthworms And Robins In A Beech Forest Makeup Which Ecological Unit?

The Mediterranean Erstwhile-Growth Forests: Anomalies or Relicts? The Contribution of Soil Charcoal Analysis
1
LIEC, CNRS, Lorraine University, 57070 Metz, France
2
Plant for Ecosystem Inquiry, CRC 1266, Kiel Academy, 24118 Kiel, Germany
3
IMBE, CNRS, IRD, Aix-Marseille Academy, Avignon University, 13290 Aix-en-Provence, France
*
Author to whom correspondence should exist addressed.
Academic Editors: Rachid Cheddadi, Adam A Ali and Cécile Remy
Received: 14 Oct 2021 / Revised: 3 November 2021 / Accustomed: five Nov 2021 / Published: 8 November 2021
Abstract
One-time-growth forests are of high interest for biodiversity conservation, especially in the disturbance-prone Mediterranean landscapes. However, it remains unclear whether the survival of patches of old-growth forest in the degraded Mediterranean landscapes results from local anomalies or from by, larger forests. Therefore, in this report, we assessed (1) the origin, (2) the long-term ecological trajectory, and (3) the machinery(s) that explain the survival of a Mediterranean old-growth forest, the Sainte-Baume forest. To achieve this, nosotros used soil charcoal analysis. We opened fifteen soil profiles in the forest and five in its surrounding areas for soil description and sampling. The soil descriptions enabled united states of america to highlight in situ soil horizon and colluvial layers. A total of 1656 charcoal pieces from different soil samples were taxonomically identified to characterize the composition of past forests. Selected charcoal pieces (n = 34) were dated to obtain chronological data. Our investigations indicate that the survival of the Mediterranean former-growth forest, in the context of the semi-open/open Mediterranean landscapes, is the result of a combination of biotic and abiotic factors, which reduced the influence of by forest disturbances. Thus, the resistance and resilience of the woods areas are preserved over a long-term ecological trajectory. Therefore, the potential of Mediterranean quondam-growth forests every bit baseline reference points for the conservation of biodiversity is related to the identification and maintenance of the local biotic and abiotic factors which allowed the survival of the old-growth forest.
1. Introduction
Old-growth forests are of high interest in terms of both biodiversity conservation [1,two,3] and climate mitigation [4,v,vi], due to their specific structure, function and limerick. This is peculiarly truthful in disturbance-prone landscapes [vii,8,9]. This is specially the case in the Mediterranean basin, in which the joint effects of ancient and significant human activities and climatic constraints accept caused the widespread presence of semi-open to open ecological systems, with as well-short disturbance return intervals to allow the development of mature wood [ten,11,12,thirteen]. Still, in this area, several isolated woodland patches have been recognized that nowadays features of old-growth forest [iii,10,fourteen,xv,16].
To date, information technology remains unclear whether the survival of these erstwhile-growth forest patches in the degraded Mediterranean landscape results from local anomalies, or whether they are the relict patches of forests that had a larger spatial distribution in the past and which take been afflicted to reach degraded wood status. This is a key question, since, in the latter case, the old-growth forests might play a meaning role for wood conservation at the Mediterranean basin scale, as a baseline reference. Therefore, it is of great importance that the origin and long-term trajectories of the Mediterranean sometime-growth forests are assessed.
However, despite a large number of paleoecological and biogeographical investigations in the area, the issue remains poorly understood. This is probably because, in the Mediterranean basin, former-growth forests are rare, isolated and restricted to small areas [3,ten]. As a consequence, their past dynamics are blurred compared with the insights garnered from the larger-scale sites studied in almost paleoecological investigations. In addition, the insights on such patches of old-growth forest are too limited in terms of spatial resolution because, in the context of the Mediterranean region, archives of records of classical paleo-indicators, such as pollen grains, are rare. Therefore, to investigate the origin and the long-term trajectory of Mediterranean old-growth forests, and the mechanisms that take enabled them to survive until today, it was necessary for u.s.a. to apply an approach that permitted the investigation of the woods's long-term history at a local scale. To this stop, we used soil charcoal records that are especially relevant at a stand scale [17,eighteen,19] and which provide local information near the by fire regime and wood composition [xx,21,22]. By conducting this investigation in an surface area of the Sainte-Baume wood with a articulate distinction betwixt a patch of old-growth wood and a matrix of degraded forest states (i.e., secondary successional forest states), a comparison was enabled of three sharply demarcated general ecological units, with the patch of one-time-growth woods surrounded past semi-open/open up ecosystems.
Inside that study area, soil samples were taken from each ecological unit for soil charcoal assay, in social club to assess (1) the origin, (2) the long-term trajectory, and (3) the mechanism(s) operating to explain the survival of the old-growth forest in the Mediterranean context of an open/semi-open mural.
2. Materials and Methods
2.1. The Report Area
The written report expanse is located on the northwestern side of the Mediterranean basin, in southern France (Figure 1a), in the calcareous Provence region [23]. The geological sub-stratum consists of limestones, from hard and compact to soft and hands erodible (i.e., marls; [24]), with karstic systems [25,26].
The climate in the written report surface area corresponds to a climate typical of Mediterranean mountains, with relatively absurd and moist winters, and warm, dry summers [27]. The local meteorological station records an average annual rainfall of 950 mm and a mean almanac temperature of 10 °C [24]. However, because the Sainte-Baume area and its surround included in the written report area, the climatic conditions may vary greatly, due to the heterogeneity of the physical factors. Indeed, the study area is composed of three dissimilar ecological units (Figure 1b) based on variations in elevation, exposure, topography, vegetation distribution and soil type.
First, to the north of the Sainte-Baume Forest is a plateau betwixt 650 and 700 m above bounding main level (a.s.fifty.). Information technology is covered mainly by pioneer coniferous woodland dominated by pine (Pinus sylvestris) and juniper (Juniperus communis and Juniperus oxycedrus). Local soils are skeletal soils, rich in calcareous stones of various sizes, corresponding to skeletic rendzic regosols.
In the center of the study area is the former-growth forest of Sainte-Baume, between 700 and 850 one thousand a.s.fifty., on a modest site at the bottom of a calcareous cliff about 300 m high. This is a mix of deciduous broadleaf and coniferous forest with mesophilic traits [24]. More precisely, at the bottom of the cliff is a showtime belt of woods several tens of meters wide, dominated past beech (Fagus sylvatica) and yew (Taxus baccata), with a few silver fir trees (Abies alba; [28]). In this office of the old-growth forest, Fagus trees have been dated past dendroecological measurements to be about 200 years old (i.e., amid 45 trees, the oldest is 249 years and the mean age is 182 years [29]).
Farther from the cliff, toward the northern plateau, is a 2nd forest belt dominated by downy oaks (Quercus pubescens) with maple (Acer spp.) and lime copse (Tilia spp.; [24]). The local soils here are more often than not deep, rich in organic matter and corresponding to cambisols.
Finally, on the southern side of the cliff, is an area of gently to locally steeply sloping land, from 900 to 650 thousand a.southward.l. There, the vegetation is a typical calcareous Mediterranean scrubland with Aleppo pine (Pinus halepensis), evergreen kermes and holm oaks (Quercus coccifera and Quercus ilex, respectively), junipers (Juniperus spp.), grey-leaved cistus (Cistus albidus), rosemary (Rosmarinus officinalis) and strawberry tree (Arbutus unedo) [24,30,31,32,33]. Local soils here are comparable with those nowadays on the northern plateau of the study surface area, i.e., rendzic regosols soils, simply also locally with deep sedimentary aggregating (mayhap greater than i m), in pocket-size stream/valley bottoms (colluvic soils), whereas, on relief ridges, soils are absent-minded, and the calcareous substrate is directly on the surface (skeletal soils).
2.2. Local History
Little is known nearly pre-historical presence in the Sainte-Baume area. Notwithstanding, in the soundings of the study expanse, several archeological sites signal the aboriginal presence of humans, such equally the Neolithic site of the "Clos des Roques" c. thirty km northeast of the Sainte-Baume, or the Mesolithic grave of Cuges-les-Pins on the west of the southern side of the Sainte-Baume cliff [34]. Other studies have shown the presence of Roman route networks, which continued the Huveaune Valley, north of the investigated plateau of the Sainte-Baume, to the ancient urban center of Marseille [35]. At the regional level, very dense occurrences of Roman structures take been found and studied, such every bit villas or oppidum [36].
During the XIII century, a monastery was constructed in the Sainte-Baume woods [37], which gave a sacred statute to the forest. Therefore, from that menstruation, the wood was protected [24]. Hunting, grazing and wood harvesting were prohibited [38]. Only later on the French Revolution, when clergy'southward country ownership was given to the local population, was the forest exploited [37,38,39,forty]. This connected until 1973, when the woods became a wildlife reserve [33]. Since 2017, the area has been included as a nature regional park, and since 2018, the forest area has been recognized nationally as "exceptional woodland".
Finally, it is interesting to note that, during the XVII and Xviii centuries, the area sur-rounding the Sainte-Baume Wood was an important source of water ice (in stone coolers), lime for lime ovens and charcoal production, with sites that are still visible today in the field [41].
2.iii. Sampling Strategy and Soil Description
The sampling strategy was designed to take account of the three ecological units of the study area (Figure 1). Nevertheless, because our inquiry focus was on the result of the quondam-growth wood, the principal effort of sampling was carried out in the Sainte-Baume forest, where 15 soil profiles were sampled along an east–west axis. To investigate the environment of the current distribution expanse of the old-growth forest, two soil profiles on the northern plateau and three soil profiles on the southern exposure of the cliff were sampled (Figure 1c).
All the soil profiles were opened manually to a length of 1–ii grand and downward to the bedrock. Each soil profile was cleaned and documented with scale drawings. Soil horizons and colluvial layers were distinguished and described co-ordinate to the backdrop observable in the field (i.e., color, texture, structure, etc. [42,43,44]). One time the soil contour had been documented, c. 10 L of soil/soil sediment was taken for charcoal extraction. The samples were taken from the base to the top of the horizon, post-obit the identified sediment layers or soil horizons, with a maximum height of 10 to xx cm per sample to maintain a fine vertical resolution of sampling [22].
2.4. Soil Charcoal Identification
Taxonomic charcoal assay was carried out on charcoal pieces larger than 1 mm which were extracted from the soil samples. We focused on these "mega-charcoal" pieces because they were large enough to be identifiable taxonomically [45], and because they were of local resolution, co-ordinate to the catchment topography [17,18,22].
The extraction of the charcoal pieces from the soil samples was carried out using the protocol described past [46,47]. Outset, the samples were wet-sieved to split the mineral fraction from the organic matter, the latter containing near of the charcoal pieces, which are low in density, floating readily. The mineral fraction was rinsed several times until the floating charcoal pieces were extracted and added to the organic fraction. Finally, the extracted organic fraction was dry-sieved using 3 different mesh sizes: 1–2 mm, two–5 mm and >5 mm.
The taxonomic identification of the charcoal pieces was performed using woods beefcake criteria, considering wood anatomy is fixed by carbonization [22]. The identification of both the genus and species level is possible if the charcoal pieces are not too muddied (e.g., covered in loamy sediment) and/or over-transformed (vitrified charcoal [48]). Identification keys, woods beefcake atlases [49,50,51] and the charcoal reference collections of the LIEC-University of Lorraine and of the IMBE-Aix-Marseille Academy were used to achieve taxonomic identification. The criteria were assessed under a stereoscopic microscope at 10× to 75× magnification and an episcopic microscope at 200×, 500× and 1000× magnifications, for upward to 90 charcoal pieces per sample, taken at random, equal proportions in each of the iii size fractions.
2.5. Charcoal Data Treatment
Considering of the mixing of soil from different layers over time [52,53,54], the results of the taxonomic analysis in this report are expressed as a proportion of taxon occurrence (i.east., taxon frequency) from the extracted charcoal assemblages per soil contour (with all depths cumulated). The absolute quantities of charcoal per sampled layer of sediment or soil horizon were non considered in this report. The only analysis related to the depth of sampling was used to compare the charcoal richness in colluvial layers vs. in situ soil horizons. For these latter data, the dry out weight of the charcoal fraction (at lx for at to the lowest degree 72 h), afterward extraction from the samples, was obtained and soil charcoal concentration was expressed relative to the dry weight of the corresponding sample, cumulated per colluvial layer and per in situ soil horizon [22].
The assay of the taxonomical data was carried out through multivariate assay. Kickoff, we used a cluster assay to wait at the frequency of every identified taxa per sampled soil profile. This cluster analysis was based on Euclidean similarity measures, proceeded on ©PAST software [55]. So, a correspondence analysis was performed of the frequencies of taxa co-ordinate to sampled soil profiles for all iii ecological units of sampling, related to the successional/autecological groups defined afterward the cluster analysis. This correspondence analysis was carried out using ©C2 software [56].
2.half dozen. Chronological Assay
Several unmarried charcoal pieces, previously taxonomically identified, were selected for radiocarbon dating. The selection of the charcoal pieces to achieve 14C dating was performed according to (i) their significance to the overall charcoal taxonomic spectrum, and (two) the context of sampling from which they were obtained, to provide chronological information about the soil erosion history (i.e., the ages of the erosion events; [57,58,59]. The carbon-14 (14C) dating was carried out by accelerator mass spectrometry (AMS) at the Laboratory of Radiocarbon Measurement of Trondheim University in Kingdom of norway (lab reference: Tra) and by the Poznań Radiocarbon Laboratory in Poland (lab reference: Poz). The radiocarbon ages were calibrated with a two-standard-deviation (2σ) 95% confidence interval on the OxCal program [60], using the IntCal20 dataset [61].
3. Results
three.1. Soil Description and Charcoal Richness
Only seven of the 20 sampled soil profiles contained in situ soil horizons, with one or two distinguishable horizons, Ah and/or Bw, covered past colluvial layers. The thirteen remaining soil profiles sampled contained just colluvial layers of varying thicknesses (from a few centimeters to several tens of centimeters; Table 1).
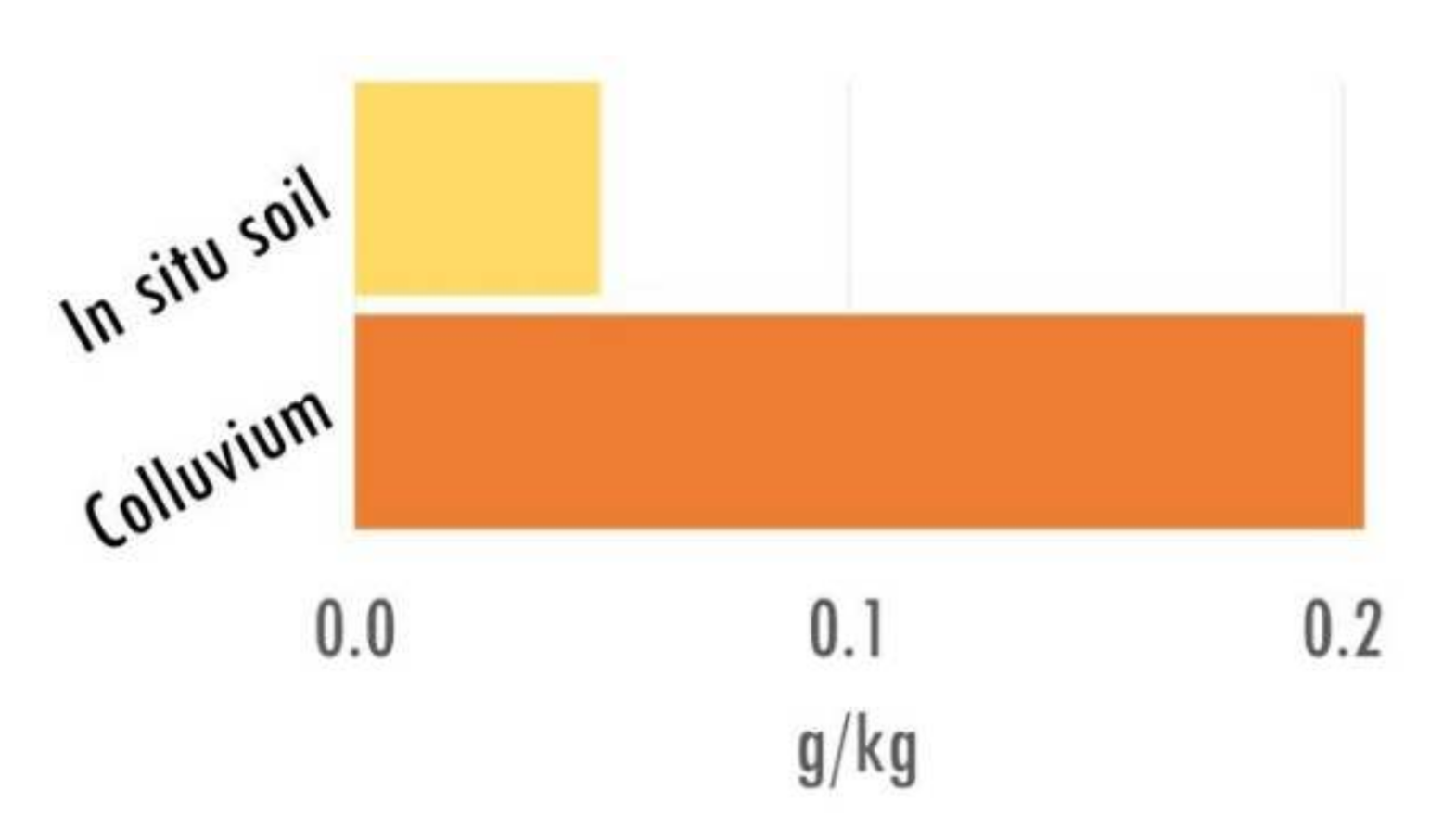
Consequently, more samples accept been taken from colluvial layers (i.e., 56 samples, providing a total of 281 kg of sediment) than from in situ soil horizons (i.e., 17 samples, providing a total of 70 kg of soil). Nonetheless, the charcoal record, expressed in concentrations (i.e., mg of charcoal >ane mm per kg of sampled soil or sediment), enabled united states of america to compare the charcoal richness of samples by the type of annal (eastward.m., soil versus soil sediment). Thus, information technology can be seen that the total charcoal concentration in the colluvial layers was more than than four times the charcoal concentration in the in situ soil horizons (Figure 2). This observation was consistent for the 3 ecological units sampled in the report area.
The absolute quantities of charcoal per sampled layer of sediment or soil horizon were non considered in this study because of the considerable biotic and abiotic heterogeneities of the sampling areas. These heterogeneities may take resulted in big variation in the previous burn regime, maybe strongly influencing the charcoal record (east.g., the blazon and amount of fuel, topography, wind exposure, etc. [62]). Moreover, the charcoal tape was not related to the depth of sampling because soils are not chronologically stratified paleoarchives. Both physical (e.yard., uprooting; [54,63]) and biological processes (east.chiliad., bioturbation; [52,64]) are potential sources of soil mixing. This appears to be particularly relevant in an area with marked relief [53], where the interpretation of chronological data from charcoal records related to depth of sampling should exist subject area to considerable caution [65].
three.2. Taxanomical Analysis
A full of 1656 charcoal pieces, from twenty soil profiles, were identified according to tree species, representing xviii different taxa. Deciduous Quercus was, by far, the well-nigh commonly identified taxon, representing 42% of the entire charcoal assemblages, followed by Fagus at 18%, Taxus and Acer at viii% each and Juniperus at 7%. All the other taxa identified were recorded at frequencies of less than one% of the whole charcoal assemblages (Table 2).
In the northern plateau, merely 45 charcoal pieces were identified, from two soil profiles. These represented four taxa: deciduous Quercus at a frequency of 40%, Pinus at 25%, Acer at twenty% and Juniperus at 16%. In the Sainte-Baume forest, from the charcoal assemblages of 15 soil profiles, the ii virtually frequent taxa, of the xi identified, were Fagus at 25% and Taxus at 15%. Less frequently occurring taxa were Acer at eight%, and other taxa such equally Tilia, Sorbus and Pinus, each nowadays at less than 3%. Finally, from the charcoal assemblages of the three soil profiles sampled on the southern exposure, the most frequent taxa, amidst the ten identified, were deciduous Quercus at 40% and Juniperus at 25%. Less oft observed taxa were evergreen Quercus at seven%, Rosmarinus at 6%, Erica and Arbutus each at 5%, Crataegus at 4% and several other taxa at frequencies below 3%.
Cluster assay of the taxonomic record for each sampled soil contour showed that the soil profiles JDF1 and JDF2 on the southern exposure were the showtime level of connection, with the third sampled profile on the southern exposure, COU1. Then, the soil profiles on the southern exposure were connected to those from the northern plateau, while just after occurred the connection to the soil profiles of the Sainte-Baume woods (Effigy 3). Cluster analysis also indicated that the identified taxa presented variable levels of similarity to one another. Thus, based on these similarities and the autecological characteristics of the identified taxa, successional groups could be formed. A first group, corresponding to the scrub (evergreen) vegetation, included Crataegus, Cistus, Pistacia, Phillyrea, evergreen Quercus and Arbutus, along with Rosmarinus, Erica and Ilex. A second group, corresponding to the pioneer taxa, included Juniperus and Pinus. A last group, corresponding to the mesophilic taxa, included Sorbus, Tilia, Acer, Ulmus and Taxus. The only taxa singled-out from these groups were Fagus and deciduous Quercus, which represented most of the charcoal records.
The correspondence assay of the taxonomic frequencies grouped by successional groups and by Fagus and deciduous Quercus show a clear pattern for each sampled soil profile (Effigy 4). On axis 1 are the sampled profiles that represent taxonomic assemblages with mesophilic affinities, plus Fagus, which represent to those taxa from the Sainte-Baume woods, distinct from communities representing scrubland vegetation, i.due east., those from the soil profiles sampled on the southern exposure. On axis 2 are the sampled profiles that stand for taxonomic assemblages with mesophilic characteristics, also every bit Fagus, which correspond to those taxa from the Sainte-Baume wood, compared to communities representing pioneer taxa from the northern plateau. Moreover, the eigenvalues of the correspondence analysis were relatively low. The axis one results explicate most 35% of the variability in the database, whereas the axis 2 results explain almost 17% of the variability in the database. This appears to be in agreement with the fact that the two different set of soil profiles, those from the northern plateau and those from the southern exposure, each represented by simply a few samples, plant the major part of the data heterogeneity, compared with the large set of soil profiles from the Sainte-Baume wood.
Finally, we tin observe that the taxa, even when grouped into successional/ecological groups, vary in their frequency depending on the ecological unit under consideration. Fagus is recorded exclusively in the samples from the Sainte-Baume forest, whereas the other mesophilic taxa are nowadays in the Sainte-Baume wood and a fiddling on the northern plateau. The pioneer taxa are mainly present on the northern plateau and the southern exposure, with merely a low frequency in the Sainte-Baume wood. The scrubland vegetation is nowadays only on the southern exposure, whereas, in dissimilarity, deciduous Quercus is nowadays in significant quantities in all three ecological units investigated (Figure v).
3.3. Chronological Analysis
A total of 34 charcoal pieces were dated by the AMS radiocarbon method. These charcoal pieces were selected from 15 of the twenty soil profiles sampled from the written report surface area. This C dating includes three dates from 2 soil profiles from the northern plateau, 20-three dates from eleven soil profiles from the Sainte-Baume Forest and eight dates from three soil profiles on the southern exposure. These dated charcoal pieces represented xv deciduous Quercus, ten Fagus, iii Juniperus, two Pinus, one Taxus, 1 Sorbus, one Crataegus and ane Arbutus (Table 3).
Fagus was the oldest dated taxon, with a date from the Mesolithic flow, while other samples were dated at different periods during the belatedly Holocene. Deciduous Quercus was dated during the belatedly Neolithic period/Statuary Age and the Centre Ages/Modern times, but with a gap from the late Bronze Historic period to Antiquity. The evergreen taxa (i.due east., Pinus, Juniperus and Arbutus), equally well as the pioneer taxa (i.e., Crataegus and Sorbus) were dated only from the tardily Holocene, with an increasing frequency from the Middle Ages to Modern times. Finally, the one date obtained for Taxus was from the belatedly Heart Ages (Figure 6).
iv. Word
four.one. Origin of the Sainte-Baume Forest
Our chronological database indicates that the oldest local record of vegetation is for Fagus at c. 8000 years before present (BP) (Table three). This record proves that Fagus had been nowadays in the Sainte-Baume wood (dated from soil profile SBA1; Effigy 1c) since the finish of the early Holocene. At a regional level, Fagus is reported at a low frequency during the early Holocene [66,67,68]. Withal, some testify for its ancient presence has been identified locally [69,70,71], supporting its record at the Sainte-Baume forest at effectually 8000 years BP. Such an aboriginal presence of Fagus, locally, might result from its early on spread from ambiguous glacial refuges in western Europe [72,73,74,75]. Subsequently, no more chronological evidence was recorded for Fagus until the belatedly Holocene. This might indicate that Fagus was not locally present anymore because of wood disturbances, peradventure due to human activities, as is the case for most of the forests of the Mediterranean basin in the mid-Holocene [x,11,12]. Yet, regional records suggest that Fagus might have remained nowadays in the long-term. Furthermore, we observed a gap without any dates for any taxa in our chronological database covering nearly the entire mid-Holocene (Effigy 6). Thus, Fagus has probably remained locally present in the Sainte-Baume woods since the end of the early on Holocene, but no burn down disturbances occurred to provide charcoal records of this taxon between c. 8000 and c. 2500 years BP.
In addition to Fagus, several other taxa with mesophilic affinities were dated in this written report, namely the records of 2 charcoal pieces of Taxus and one of Sorbus, all from the ecological unit of the Sainte-Baume wood. These charcoal pieces were dated back to the tardily Holocene (Table iii). The presence of Taxus supports several additional sources of evidence for this taxon in the region over the same period, related notably to late Neolithic settlements [76,77,78]. These late Holocene records are in accordance with our record of Fagus from the aforementioned period. Taken together, these records bespeak the presence of a mesophilic mixed forest since at least the late Holocene on the site of the Sainte-Baume forest.
Thus, co-ordinate to our chronological evidence, the origin of the Sainte-Baume woods goes back to Roman development during Antiquity or to the institution of a monastery on the area during the High Heart Ages (AD 1000 to 1250), every bit had been postulated previously [24,79,fourscore,81].
Deciduous Quercus were also commonly present in the charcoal assemblages from all the sampled ecological units (Figure v). This deciduous oak is very probable to be Quercus pubescens, since this is the only Mediterranean deciduous oak species present in the calcareous soils of Provence. Its presence at a regional level in the Mediterranean landscapes has been attested to since at to the lowest degree the mid-Holocene [69,71,82]. This is in agreement with our tape for this taxon, which has been locally present since c. 4500 years BP on the northern plateau, since c. 4000 years BP in the Sainte-Baume wood and since c. 3500 years BP on the southern exposure, with relatively frequent occurrences later on until Modern times (Figure 6). Therefore, we postulate that deciduous Quercus, most likely Q. pubescens, was present, since the mid Holocene, in the Sainte-Baume forest, maybe in combination with Fagus and other taxa with mesophilic affinities. Moreover, deciduous Quercus was besides present in the areas surrounding the Sainte-Baume forest, as confirmed by the dates nosotros obtained for this taxon from charcoal samples from the northern plateau and the southern exposure of the Sainte-Baume forest, dating from the mid- to the belatedly Holocene (Figure 6, Table 3).
4.2. The Long-Term Trajectory of the Sainte-Baume Woods and Its Environment
Analysis of the charcoal assemblages from the study area clearly indicates different taxa comprising the vegetation from the various ecological units sampled (Figure 3 and Effigy iv).
The samples from the Sainte-Baume forest show taxonomic groupings closely resembling the present-day vegetation in the area, of a mesophilic mixed forest. Thus, it seems that the ecological trajectory of the Sainte-Baume forest did not change in the long term, or did not modify sufficiently to induce meaning changes in forest limerick. Indeed, the presence of pioneer taxa in the local charcoal assemblages, such as Juniperus and Pinus (Figure v), indicates the occurrence of on-site burn disturbances. This is supported by the frequent presence of colluvial layers in the soil profiles of the Sainte-Baume forest, which contained well-nigh of the charcoal records (Figure 2), and indicate the well-recognized coupled mechanisms of fire and postal service-burn down erosion [57,59,83]. Even so, these disturbance events did non generate large woods openings, thereby preventing large-calibration soil erosion and subsequent meaning changes in the long-term woods trajectory.
The charcoal assemblages of the samples from the northern plateau evidence little evidence of mesophilic taxa but more pioneer taxa (Effigy 5). This finding is consistent with regional woods openings and ingather development since the Neolithic period in the Mediterranean regions [12,84,85]. Notwithstanding, the number of sampled soil profiles in the northern plateau is too low to exclude the possible occurrences of other vegetation types in that area. For the more than recent past, written sources assert that forest harvesting occurred on the northernmost belt of the Sainte-Baume forest during the French Revolution [24,37]. These forest openings might explain the shift from the mesophilic forest to the pioneer woods on the northern plateau, resulting today in a mixed deciduous Quercus/Pinus woods. However, the only mesophilic taxon found on the northern plateau is Acer spp. This taxonomic record might represent to a Mediterranean Acer such as Acer monspessulanum, which is present on-site today. The mail-pioneer autecology of Acer spp. [86] might also explain its presence on the northern plateau, in pioneer-dominated charcoal collections.
The tape of deciduous Quercus (cf. Q. pubescens) in the charcoal assemblages on the northern plateau, besides as in those from the Sainte-Baume forest and the southern exposure, indicates the cracking importance of this tree over the entire study area. This agrees with the report that Q. pubescens-dominated woodlands constituted a considerable part of the Mediterranean landscapes in the by [10,11,12], at least until they became heavily degraded by man activities, such equally pastoralism, combined with anthropogenic fires, since the Neolithic, and especially during the Roman Historic period and onward [13,84,87,88]. Therefore, information technology is particularly interesting to note that our chronological database and our charcoal taxonomic analysis assemblages indicate that deciduous Quercus/Q. pubescens remains nowadays in the long-term woods trajectory of the Sainte-Baume forest and its surroundings until today. Deciduous Quercus/Q. pubescens is, notwithstanding, absent-minded today from the southern exposure of the Sainte-Baume forest. Nonetheless, it is notably present in the historical local charcoal collections (Effigy 5), with the chronological data indicating its presence on-site at to the lowest degree c. 500 years ago, and far back equally c. 3500 years BP (Tabular array 3). The latter, more ancient, record for this taxon on the southern exposure appears after than those of the Sainte-Baume forest and the northern plateau (Table 3), and even than the Holocene dynamics of this taxon [69,71,82]. The locally strong erosive processes associated with local past land use and steep slopes (Figure ane) probably erased the early on and mid-Holocene communities at the sampling locations. In add-on, the number of sampled soil profiles was low on the southern exposure of the Sainte-Baume forest. Thus, it does not allow the exclusion of other, peradventure older, vegetation records. Withal, on this latter ecological unit, the taxonomic limerick of the charcoal assemblages was very like to the current local vegetation, except for deciduous Quercus. This is a articulate, semi-open up woodland, with xeric, pioneer/mail-pioneer vegetation, with, in the contempo past (c. 500 years ago), the presence of deciduous Quercus/Q. pubescens. This fits with the "classical" Mediterranean long-term vegetation dynamics following land use, soil erosion and climate pejoration [13,84,87,88].
4.3. The Mechanism(s) of Survival of the Old-Growth Sainte-Baume Forest
Our investigations indicate out that the origin of the Sainte-Baume forest and its long-term trajectory evidence pregnant locally specific features, in contrast with the vegetation trajectory of the surrounding area (Figure 1b). Indeed, the charcoal assemblages and their chronology are significantly different from those from the northern plateau and the southern exposure, with the presence of taxa with mesophilic affinities in the Sainte-Baume woods (Figure 5 and Figure six). The found communities from the northern plateau and the southern exposure prove some similarities in vegetation limerick, although they also present some differences, with mostly pioneer species on the northern plateau and evergreen scrub on the southern exposure (Table 2 and Figure 3). This finding is supported by the eigenvalues of the correspondence assay, which are relatively depression (Figure 4). This is in agreement with the fact that the charcoal assemblages from the soil samples from the northern plateau and the southern exposure constitute the major function of the data heterogeneity, fifty-fifty though both sites are represented by only a few samples, compared with the large set of soil profiles obtained from the Sainte-Baume woods. Therefore, the past vegetation limerick and dynamics of the Sainte-Baume woods, since its origin at least c. 3000 years BP and its subsequent long-term trajectory, has been very local, as it is today, as a patch of temperate wood in a matrix of semi-open/open ecosystems.
To explicate the survival of this forest patch, several hypotheses have been postulated, related to local abiotic factors or anthropological influences [24,twoscore]. Our investigations suggest that, indeed, local abiotic factors and anthropogenic influences played a meaning role in the survival of the Sainte-Baume wood over a long-term ecological trajectory until the present twenty-four hours. It appears that the origin of the forest and its development until the mid-Holocene were probably closely related to the appropriate local abiotic conditions caused past the presence of the cliff. This specific factor was, and all the same is, favorable for the occurrence of temperate ecology conditions, in contrast to the southern exposure of the Sainte-Baume forest, on which the forest vegetation seems to take been degraded early in time to create open up/semi-open states, as has been demonstrated in many areas of the Mediterranean region [10,11,12,xiii].
While the establishment of the monastery during the Middle Ages does not explain the origin of the Sainte-Baume forest, its long-term survival and development is probably a result of the sacred statute that limited the use of wood resources [38]. The existence of a sacred statute permitted the maintenance of the ancient, natural, mesophilic Sainte-Baume wood every bit the remarkable former-growth forest it is today.
However, the charcoal record and colluvial layers from within the Sainte-Baume forest (Table 1 and Figure 2) attest to the fact that the forest was subjected to regular local fires and soil erosion events. The chronology of these local events of wood disturbances (Table three and Effigy half-dozen) fit well with the strong stage of human being impact on the Mediterranean ecosystems which occurred during the late Holocene [87,88,89,90]. However, in the Sainte-Baume forest, these forest disturbances did non cause the opening-up of the forest and subsequent soil degradation, and thus did not cause changes in vegetation limerick in the long term. Therefore, we postulate that the by woods disturbances in the Sainte-Baume forest were only local (i.eastward., at stand scale) and occurred with a high render interval. This was due probably to the fact that the Sainte-Baume woods exhibited (one) a high ecological resistance to disturbances, at a large calibration, thanks to the protection from the monastery, and (two) a high resilience at the local scale thanks to the favorable local environmental conditions caused by the presence of the cliff.
5. Conclusions
The paleoecological approach, based on local indicators, applied to the current study permitted the identification of the origin of the Sainte-Baume Woods as being in the mid-Holocene, at least. Moreover, in the same time window, we evidence that the surroundings of the woods area, at least the northern plateau and the southern exposure of the Sainte-Baume woods, were in very different states in terms of vegetation composition, respective to degraded wood landscapes. Thus, it is clear that, if the modern old-growth Saint-Baume forest is a relict patch of woods with mesophilic affinities, its origin is related to very erstwhile natural vegetation dynamics, and its institution from the mid-Holocene to the late Holocene is probably related to a local specific cistron, namely the influence of the cliff. This local relief also played a part in the long-term survival of the Sainte-Baume forest, as a mixed deciduous broadleaf–conifer forest with mesophilic traits. Indeed, our approach immune united states to highlight forest fire disturbances, and the resistance and the resilience of the forest system, which limited the impact of these disturbances on the local scale and over short time periods, without change in the forest state in the long term and on a large calibration. In the finish, the mechanisms that have conferred such resistance and resilience to the Sainte-Baume wood have been identified equally (1) the forest protection cheers to the presence of a monastery since the early Heart Ages that conferred a sacred statute to the woods, and (2) the local temperate environmental weather condition on the north side of the cliff.
To conclude, the investigations conducted on this case study of the Sainte-Baume old-growth forest bespeak that the survival of the Mediterranean old-growth forests, in the context of the semi-open/open up landscapes of the Mediterranean basin, results from a combination of favorable biotic and abiotic factors. Although this combination of factors probably differs locally, it plays a role past limiting the influence of the disturbances which might impact on the forest surface area, possibly on different temporal scales. In this way, the resistance and the resilience of the forest areas are preserved on a long-term ecological trajectory. Therefore, if information technology is true that the Mediterranean old-growth forests are of prime importance for the conservation of forest biodiversity, their potential as a baseline reference state is strongly limited by the spatial scale of the biotic and abiotic factors involved as a mechanism to explain the survival of the old-growth forests. Therefore, the potential of the Mediterranean old-growth forests to human activity as a reference is related to the identification and maintenance of the local biotic and abiotic factors which permit the survival of the old-growth forests.
Author Contributions
Conceptualization, 5.R. and B.T.; methodology, V.R., S.D. and B.T.; investigation, V.R., S.D. and B.T.; resources, 5.R.; writing—original typhoon preparation, V.R.; writing—review and editing, Due south.D. and B.T. All authors have read and agreed to the published version of the manuscript.
Funding
This enquiry received no external funding.
Acknowledgments
We are thankful to the members of the local forest agency, especially François Ferraina, and to Jacob Defarafeibona and Hannes Knapp for their participation in the field work.
Conflicts of Involvement
The authors declare no disharmonize of interest.
References
- Hilbert, J.; Wiensczyk, A. Old-growth definitions and direction: A Literature review. J. Ecosyst. Manag. 2007, 8, 15–31. [Google Scholar]
- Wirth, C.; Gleixner, G.; Heimann, Yard. Old-Growth Forests: Function, Fate and Value. In Ecological Studies 207; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Mansourian, S.; Rossi, M.; Vallauri, D. Ancient Forests in the Northern Mediterranean: Neglected High Conservation Value Areas; WWF: Marseille, France, 2013. [Google Scholar]
- Luyssaert, S.; Detlef, South.East.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.Eastward.; Ciais, P.; Grace, J. Sometime-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, J.C.; Thompson, J.R.; Epstein, H.E.; Shugart, H.H., Jr. Carbon storage in old-growth forests of the Mid-Atlantic: Toward better agreement the eastern forest carbon sink. Ecology 2015, 96, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Bong, D.M.; Kim, H.; Nelson, P.North.; Schulze, M.; Betts, M.Thou. Temporal consistency of undercanopy thermal refugia in sometime-growth forest. Agric. For. Meteorol. 2021, 307, 108520. [Google Scholar] [CrossRef]
- Spies, T.A.; Hemstrom, Thousand.A.; Youngblood, A.; Hummel, S. Conserving Old-Growth Forest Diversity in Disturbance-Prone Landscapes. Conserv. Biol. 2006, 20, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, C.Due east.; Friederici, P.; Petruncio, M. Monitoring old growth in frequent-burn down landscapes. Ecol. Soc. 2007, 12, 22. Available online: http://www.ecologyandsociety.org/vol12/iss2/art22 (accessed on 5 Nov 2021). [CrossRef]
- De Assis Barros, 50.; Elkin, C. An index for tracking old-growth value in disturbance-prone forest landscapes. Ecol. Indic. 2021, 121, 107175. [Google Scholar] [CrossRef]
- Quézel, P.; Médail, F. Écologie et Biogéographie des Forêts du Bassin Méditerranéen; Elsevier: Paris, France, 2003. [Google Scholar]
- Grove, A.T.; Rackham, O. The Nature of Mediterranean Europe: An Ecological History; Yale University Press: New Haven, UK, 2003. [Google Scholar]
- Blondel, J.; Aronson, J.; Bodiou, J.-Y.; Bœuf, G. The Mediterranean Region. Biological Diversity in Infinite and Time; Oxford University Press: Oxford, U.k., 2010. [Google Scholar]
- Mercuri, A.M.; Florenzano, A.; Burjachs, F.; Giardini, M.; Kouli, K.; Masi, A.; Picornell-Gelabert, L.; Revelles, J.; Sadori, L.; Servera-Vives, G.; et al. From influence to impact: The multifunctional country use in Mediterranean prehistory emerging from palynology of archaeological sites (eight.0–two.8 ka BP). Holocene 2019, 29, 830–846. [Google Scholar] [CrossRef]
- Chirici, G.; Nocentini, Southward. Old-growth forests in Italy: Contempo research developments and future perspectives. L'Italia For. Due east Mont. 2010, 65, 475–480. [Google Scholar] [CrossRef]
- Lombardi, F.; Chirici, G.; Marchetti, M.; Tognetti, R.; Lasserre, B.; Corona, P.; Barbati, A.; Ferrari, B.; Di Paolo, S.; Giuliarelli, D.; et al. Deadwood in wood stands shut to old-growthness nether Mediterranean weather condition in the Italian Peninsula. L'Italia For. East Mont. 2010, 65, 481–584. [Google Scholar] [CrossRef]
- Badalamenti, E.; La Mantia, T.; La Mantia, G.; Cairone, A.; Veca, D.Due south.L.M. La Mantiving and Dead Aboveground Biomass in Mediterranean Forests: Testify of One-time-Growth Traits in a Quercus pubescens Willd. s.l. Stand up. Forests 2017, eight, 187. [Google Scholar] [CrossRef]
- Dutoit, T.; Thinon, Thousand.; Talon, B.; Buisson, Due east.; Alard, D. Sampling soil wood charcoals at a high spatial resolution: A new methodology to investigate the origin of grassland constitute communities. J. Veg. Sci. 2009, 20, 349–358. [Google Scholar] [CrossRef]
- Touflan, P.; Talon, B.; Walsh, 1000. Soil charcoal analysis: A reliable tool for spatially precise studies of past wood dynamics: A case study in the French southern Alps. Holocene 2010, 20, 45–52. [Google Scholar] [CrossRef]
- Feiss, T.; Horen, H.; Brasseur, B.; Lenoir, J. Optimal sampling design and minimal effort for soil charcoal analyses considering the soil blazon and wood history. Veg. Hist. Archaeobotany 2017, 26, 627–637. [Google Scholar] [CrossRef]
- Gavin, D.1000. Interpretation of inbuilt age in radiocarbon ages of soil charcoal for fire history studies. Radiocarbon 2001, 43, 27–44. [Google Scholar] [CrossRef]
- Fesenmyer, K.A.; Christensen, N.L. Reconstructing Holocene fire history in a southern Appalachian forest using soil charcoal. Ecology 2010, 91, 662–670. [Google Scholar] [CrossRef]
- Robin, 5.; Bork, H.-R.; Nadeau, Grand.-J.; Nelle, O. Fire and forest history of primal European depression mountain forest sites based on soil charcoal analysis: The example of the eastern Harz. Holocene 2014, 24, 35–47. [Google Scholar] [CrossRef]
- IFN. Une nouvelle partition écologique et forestière du territoire métropolitain : Les sylvoécorégions (SER). Bull. L'if 2011, 26, ane–8. [Google Scholar]
- Darras, C.; Tresmontant, D.; Lemaire, C. La Forêt Sacrée de la Sainte-Baume; Naturalia Publications: Turriers, French republic, 2017. [Google Scholar]
- Blanc, J.-J. Histoire des creusements karstiques et des surfaces d'érosion en Provence occidentale. Physio Géo. 2010, 4, one–26. [Google Scholar] [CrossRef]
- Mazet, J.; Nicod, J. Les bassins supérieurs du Cauron et du Caramy, au nord-est du massif de la Sainte Baume (Var, Provence): Des hydrosystèmes karstiques complexes. Études Géographie Phys. 2012, 39, 21–51. [Google Scholar]
- Vila, B.; Vennetier, M.; Ripert, C.; Chandioux, O.; Liang, E.; Guibal, F.; Torre, F. Has global change induced divergent trends in radial growth of Pinus sylvestris and Pinus halepensis at their bioclimatic limit? The example of the Sainte-Baume forest (south-due east France). Ann. For. Sci. 2008, 65, 709. [Google Scholar] [CrossRef]
- Charles, J.P. Une hêtraie à Androsace chaixii dans le massif de la Sainte-Baume. Bull. Soc. Linn. Provence 1991, 42, 59–seventy. [Google Scholar]
- Cattenoz, D. Dynamiques Passée et Actuelle de la Hêtraie de la Sainte-Baume (Var). Master'southward Thesis, Aix-Marseille University, Aix-en-Provence, French republic, 2009. [Google Scholar]
- Molinier, R. Flore de la forêt domaniale de la Sainte Baume (Var). Catalogue des espèces présentes dans les limites de la forêt. Ann. Soc. Nat. Archéol. ToulonVar. 1950, 3, 45–66. [Google Scholar]
- Bonin, Grand.; Gamisans, J.; Gruber, Yard. Etude des successions dynamiques de la végétation de la Sainte Baume. Ecol. Mediterr. 1983, 9, 129–171. [Google Scholar] [CrossRef]
- Bonin, Chiliad.; Sandoz, H.; Thinon, Yard.; Vedrenne, G. Relations entre la dynamique de la végétation (chênaie hêtraie) et les caractéristiques édaphiques dans le massif de la Sainte-Baume (Provence). Ecol. Mediterr. 1984, 9, 193–210. [Google Scholar] [CrossRef]
- Quertier, P.; Guicheteau, D. Site Natura 2000 PR 110–FR 9301606 Massif de la Sainte Baume—Document d'Objectifs Natura 2000; Muséum National d'Histoire Naturelle: Paris, France, 2001. [Google Scholar]
- SRA. Bilan Scientifique de la Région Provence-Alpes-Côte d'Azur; Ministère de la Civilisation et de la Communication, Management Générale des Patrimoines, Sous-Direction de l'Archéologie, Service Regional d'Archeologie: Aix-en-Provence, France, 2013. [Google Scholar]
- Servan, P. Voies antiques de la moyenne vallée de l'Huveaune. Rev. Provence Hist. 1960, 10, 189–221. [Google Scholar]
- Brun, J.-P.; Conges, 1000.; Geraba, C.; Pasqualini, Yard. L'habitat rural dans le Var à l'époque romaine: Données archéologiques récentes. Rev. Provence Hist. 1985, 35, 233–251. [Google Scholar]
- Fedele, A. From Christian religion to feminist spirituality: Mary Magdalene pilgrimages to La Sainte-Baume, France. Cult. Relig. 2009, 10, 243–261. [Google Scholar] [CrossRef]
- Chalvet, M. La forêt domaniale de la Sainte-Baume : Un espace exceptionnel et protégé en Provence. Cahiers Framespa 2013, 13, i–19. [Google Scholar] [CrossRef]
- Hervé, P. La forêt domaniale de la Sainte-Baume (Var)—Problèmes posés par sa gestion. Rev. For. Française 1953, 9, 557–564. [Google Scholar] [CrossRef]
- Dugelay, A. La Sainte-Baume. Esquisse d'histoire d'une relique forestière. Rev. Bois mars. 1957. [Google Scholar]
- Nédonsel, Y. Le massif de la Sainte Baume et la production de glace naturelle: Les glacières de Fontfrège. Rev. Régionale D'ethnologie 1981, 2–3, 103–125. [Google Scholar] [CrossRef]
- Munsell. Soil Colour Charts; Gretag Macbeth: New Windsor, NY, USA, 2000. [Google Scholar]
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C. Soil Survey Staff. Field Book for Describing and Sampling Soils, Version ii.0; U.S. Department of Agriculture, Natural Resource Conservation Service, National Soil Survey Centre: Lincoln, NE, USA, 2002. [Google Scholar]
- Sponagel, H.; Grottenthaler, West.; Hartmann, One thousand.J.; Eckelmann, W. Bodenkundliche Kartieranleitung (fifth verbesserte und erweiterte Auflage); Bundesanstalt für Geowissenschaften und Rohstoffe: Hannover, Germany, 2005. [Google Scholar]
- Robin, V.; Nadeau, One thousand.-J.; Grootes, P.Thou.; Bork, H.; Nelle, O. Too early on and too northerly: Evidence of temperate trees in northern Fundamental Europe during the Younger Dryas. New Phytol. 2016, 212, 259–268. [Google Scholar] [CrossRef]
- Carcaillet, C.; Thinon, M. Pedoanthracological contribution to the study of the evolution of the upper treeline in the Maurienne Valley (N French Alps): Methodology and preliminary data. Rev. Palaeobot. Palynol. 1996, 91, 399–416. [Google Scholar] [CrossRef]
- Talon, B.; Carcaillet, C.; Thinon, M. Études pédoanthracologiques des variations de la limite supérieure des arbres au cours de l'Holocène dans les Alpes Françaises. Géographie Phys. Quat. 1998, 52, 195–208. [Google Scholar] [CrossRef]
- McParland, L.C.; Collinson, One thousand.E.; Scott, A.C.; Campbell, Thou.; Veal, R. Is vitrification in charcoal a result of high temperature called-for of forest? J. Archaeol. Sci. 2010, 37, 2679–2687. [Google Scholar] [CrossRef]
- Wheeler, E.A.; Baas, P.; Gasson, P.E. IAWA list of microscopic features for hardwood identification. IAWA Balderdash. 1989, 10, 219–332. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Anatomy of European Woods; Haupt Verlag AG: Bern, Switzeralnd,, 1990. [Google Scholar]
- Schweingruber, F.H. Microscopic Wood Anatomy; Swiss Federal Establish for Forest, Snow and Mural Inquiry: Birmensdorf, Switzeralnd, 1990. [Google Scholar]
- Darwin, C. The Germination of Vegetable Mould, Through the Action of Worms, with Observations on their Habits. John Murray: London, U.k., 1881. [Google Scholar]
- Carcaillet, C. Are Holocene wood-charcoal fragments stratified in alpine and subalpine soils? Show from the Alps based on AMS 14C dates. Holocene 2001, 11, 231–242. [Google Scholar] [CrossRef]
- Šamonil, P.; Král, K.; Hort, L. The role of tree uprooting in soil formation: A critical literature review. Geoderma 2010, 157, 65–79. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Parcel for Teaching and Data Analysis. Palaeontol. Electron. 2001, four, 9. [Google Scholar]
- Juggins, South. User Guide C2—Software for Ecological and Palaeoecological. Data Analysis and Visualization User Guide, version 1.5; University of Newcastle: Newcastle, NWS, Australia, 2007. [Google Scholar]
- Bork, H.-R.; Lang, A. Quantification of past erosion and land use/land embrace changes in Germany. In Long Term Hillslope and Fluvial Organisation Modelling; Lang, A., Hennrich, K., Dikan, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 231–239. [Google Scholar]
- Leopold, K.; Völkel, J. Colluvium: Definition, differentiation, and possible suitability for reconstructing Holocene climate data. Quat. Int. 2007, 162–163, 133–140. [Google Scholar] [CrossRef]
- Dreibrodt, S.; Lubos, C.; Terhorst, B.; Damm, B.; Bork, H.-R. Historical soil erosion past water in Frg: Scales and archives, chronology, research perspectives. Quat. Int. 2010, 222, 80–95. [Google Scholar] [CrossRef]
- Bronk-Ramsey, C.; Lee, S. Recent and Planned Developments of the Program OxCal. Radiocarbon 2013, 55, 720–730. [Google Scholar] [CrossRef]
- Reimer, P.J.; Bard, E.; Bayliss, A.; Beck, J.West.; Blackwell, P.G.; Ramsey, C.B.; Buck, C.E.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. INTCAL13 and MARINE13 radiocarbon historic period calibration curves 0-50,000 years cal BP. Radiocarbon 2013, 55, 1869–1887. [Google Scholar] [CrossRef]
- Whitlock, C.; Higuera, P.; McWethy, D.B.; Briles, C.E. Paleoecological Perspectives on Burn Ecology: Revisiting the Fire-Authorities Concept. Open Ecol. J. 2010, 3, vi–23. [Google Scholar] [CrossRef]
- Gavin, D.Thousand. Forest soil disturbance intervals inferred from soil charcoal radiocarbon dates. Tin can. J. For. Res. 2003, 33, 2514–2518. [Google Scholar] [CrossRef]
- Jégou, D.; Cluzeau, D.; Balesdent, J.; Tréhen, P. Effects of four ecological categories of earthworms on carbon transfer in soil. Appl. Soil Ecol. 1998, 9, 249–255. [Google Scholar] [CrossRef]
- Talon, B. Reconstruction of Holocene high altitude vegetation cover in the French Southern Alps: Show from soil charcoal. Holocene 2010, 20, 34–44. [Google Scholar] [CrossRef]
- Giesecke, T.; Hickler, T.; Kunkel, T.; Sykes, M.T.; Bradshaw, R.H.; Hickler, T.; Kunkel, T.; Sykes, R.H.; Bradshaw, R.H. Towards an understanding of the Holocene distribution of Fagus sylvatica L. J. Biogeogr. 2007, 34, 118–131. [Google Scholar] [CrossRef]
- Magri, D. Patterns of mail service-glacial spread and the extent of glacial refugia of European beech (Fagus sylvatica). J. Biogeogr. 2008, 35, 450–463. [Google Scholar] [CrossRef]
- Bradshaw, R.H.W.; Kito, N.; Giesecke, T. Factors influencing the Holocene history of Fagus. For. Ecol. Manag. 2010, 259, 2204–2212. [Google Scholar] [CrossRef]
- Laval, H.; Medus, J.; Roux, M. Palynological and sedimentological records of Holocene human impact from the Etang de Berre, southeastern France. Holocene 1991, i, 269–272. [Google Scholar] [CrossRef]
- Delhon, C.; Thiébault, South.; Brochier, J.-L.; Berger, J.-F. Dynamiques de végétation au Tardiglaciaire et à l'Holocène ancien en moyenne vallée du Rhône d'après les données anthracologiques. Quaternaire 2010, 21, 281–293. [Google Scholar] [CrossRef]
- Azuara, J.; Lebreton, V.; Peyron, O.; Mazier, F.; Combourieu-Nebout, N. The Holocene history of low altitude Mediterranean Fagus sylvatica forests in southern France. J. Veg. Sci. 2018, 29, 438–449. [Google Scholar] [CrossRef]
- Delhon, C.; Thiébault, S. The migration of beech (Fagus sylvatica L.) upwardly the Rhone: The Mediterranean history of a "mountain" species. Veg. Hist. Archaeobotany 2005, 14, 119–132. [Google Scholar] [CrossRef]
- De Lafontaine, 1000.; Guerra, C.A.A.; Ducousso, A.; Petit, R.J. Cryptic no more: Soil macrofossils uncover Pleistocene wood microrefugia within a periglacial desert. New Phytol. 2014, 204, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Robin, 5.; Knapp, H.; Rickert, B.; Talon, B.; Nelle, O. Comparaison de signaux anthracologiques Holocènes issus de différents types d'athenaeum en Allemagne vers une reconstitution plus précise de 50'histoire des incendies ? Quaternaire 2013, 24, 167–177. [Google Scholar] [CrossRef]
- Stewart, J.R.; Lister, A.M. Cryptic northern refugia and the origins of the modern biota. Trends Ecol. Evol. 2001, 16, 608–613. [Google Scholar] [CrossRef]
- Henry, F.; Talon, B.; Dutoit, T. The age and the history of the French Mediterranean steppe revisited past soil woods charcoal analysis. Holocene 2010, twenty, 25–34. [Google Scholar] [CrossRef]
- Casals, P.; Camprodon, J.; Caritat, A.; Rios, A.I.; Guixé, D.; Garcia-Marti, Ten.; Martin-Alcon, S.; Coll, L. Forest structure of Mediterranean yew (Taxus baccata 50.) populations and neighbor effects on juvenile yew functioning in the NE Iberian Peninsula. For. Syst. 2015, 24, e042. [Google Scholar] [CrossRef]
- Iszkuło, G.; Pers-Kamczyc, Due east.; Nalepka, D.; Rabska, Thousand.; Walas, Łukasz; Dering, K. Postglacial migration dynamics helps to explain current scattered distribution of Taxus baccata. Dendrobiology 2016, 76, 81–89. [Google Scholar] [CrossRef]
- Guinier, P. Plaidoyer pour la Sainte-Baume auprès de Monsieur le Juge de Paix de Saint-Maximin. Dacty 1944. [Google Scholar]
- Molinier, R. La hêtraie de la forêt domaniale de la Sainte-Baume. Bull. MNHN Marseille 1952, 12, 63–85. [Google Scholar]
- Thiébaut, B. Étude Ecologique de la Hêtraie dans fifty'Arc Montagneux Nord Méditerranéen de la Vallée du Rhône à Celle de 50'Ebre. PhD Thesis, Languedoc University, Montpellier, France, 1976. [Google Scholar]
- Nicol-Pichard, S. Analyse pollinique d'une séquence Tardiet Postglaciaire à Tourves (var, France). Ecol. Med. 1987, 13, 29–42. [Google Scholar]
- Capitanio, R.; Carcaillet, C. Post-fire Mediterranean vegetation dynamics and multifariousness: A discussion of succession models. For. Ecol. Manag. 2008, 255, 431–439. [Google Scholar] [CrossRef]
- Barbero, M.; Bonin, G.; Loisel, R.; Quézel, P. Changes and disturbances of forest ecosystems caused past human being activities in the western part of the Mediterranean basin. Vegetatio 1990, 87, 151–173. [Google Scholar] [CrossRef]
- Roberts, C.N.; Woodbridge, J.; Palmisano, A.; Bevan, A.; Fyfe, R.; Shennan, S. Mediterranean landscape change during the Holocene: Synthesis, comparison and regional trends in population, land cover and climate. Holocene 2019, 25, 923–937. [Google Scholar] [CrossRef]
- Dumé, Grand.; Gauberville, C.; Mansion, D.; Rameau, J.-C. Flore Forestière Française; Institut pour le développement forestier, Centre national de la propriété forestière: Paris, France, 2003. [Google Scholar]
- Jalut, G.; Dedoubat, J.J.; Fontugne, M.; Otto, T. Holocene circum-Mediterranean vegetation changes: Climate forcing and human being touch on. Quat. Int. 2009, 200, 4–18. [Google Scholar] [CrossRef]
- Berger, J.-F.; Shennan, South.; Woodbridge, J.; Palmisano, A.; Mazier, F.; Nuninger, L.; Guillon, S.; Doyen, East.; Begeot, C.; Andrieu-Ponel, V.; et al. Holocene land cover and population dynamics in Southern France. Holocene 2019, 25, 776–798. [Google Scholar] [CrossRef]
- Butzer, Grand.W. Environmental history in the Mediterranean earth: Cross-disciplinary investigation of cause-and-result for degradation and soil erosion. J. Archaeol. Sci. 2005, 32, 1773–1800. [Google Scholar] [CrossRef]
- Vannière, B.; Colombaroli, D.; Chapron, E.; Leroux, A.; Tinner, W.; Magny, Chiliad. Climate versus human-driven fire regimes in Mediterranean landscapes: The Holocene record of Lago dell'Accesa (Tuscany, Italy). Quat. Sci. Rev. 2008, 27, 1181–1196. [Google Scholar] [CrossRef]
Figure 1. Presentation of the report surface area: (a) location of the Sainte-Baume forest area; (b) topographical profile of the Sainte-Baume Massif on the northward–south axis passing through the so-called "hotellerie", the Saint-Pilon Laissez passer and the Vallon de Trébuquet. The scarlet borders mark the three ecological units in the study expanse (A: northern plateau; B: Sainte-Baume woods; and C: southern exposure); (c) location of the sampled soil contour in the report surface area (red dots) (used maps under GNU General Public Licenses).
Figure one. Presentation of the report surface area: (a) location of the Sainte-Baume forest surface area; (b) topographical profile of the Sainte-Baume Massif on the north–southward axis passing through the and then-called "hotellerie", the Saint-Pilon Pass and the Vallon de Trébuquet. The cherry-red borders mark the iii ecological units in the study area (A: northern plateau; B: Sainte-Baume forest; and C: southern exposure); (c) location of the sampled soil profile in the study expanse (red dots) (used maps nether GNU General Public Licenses).
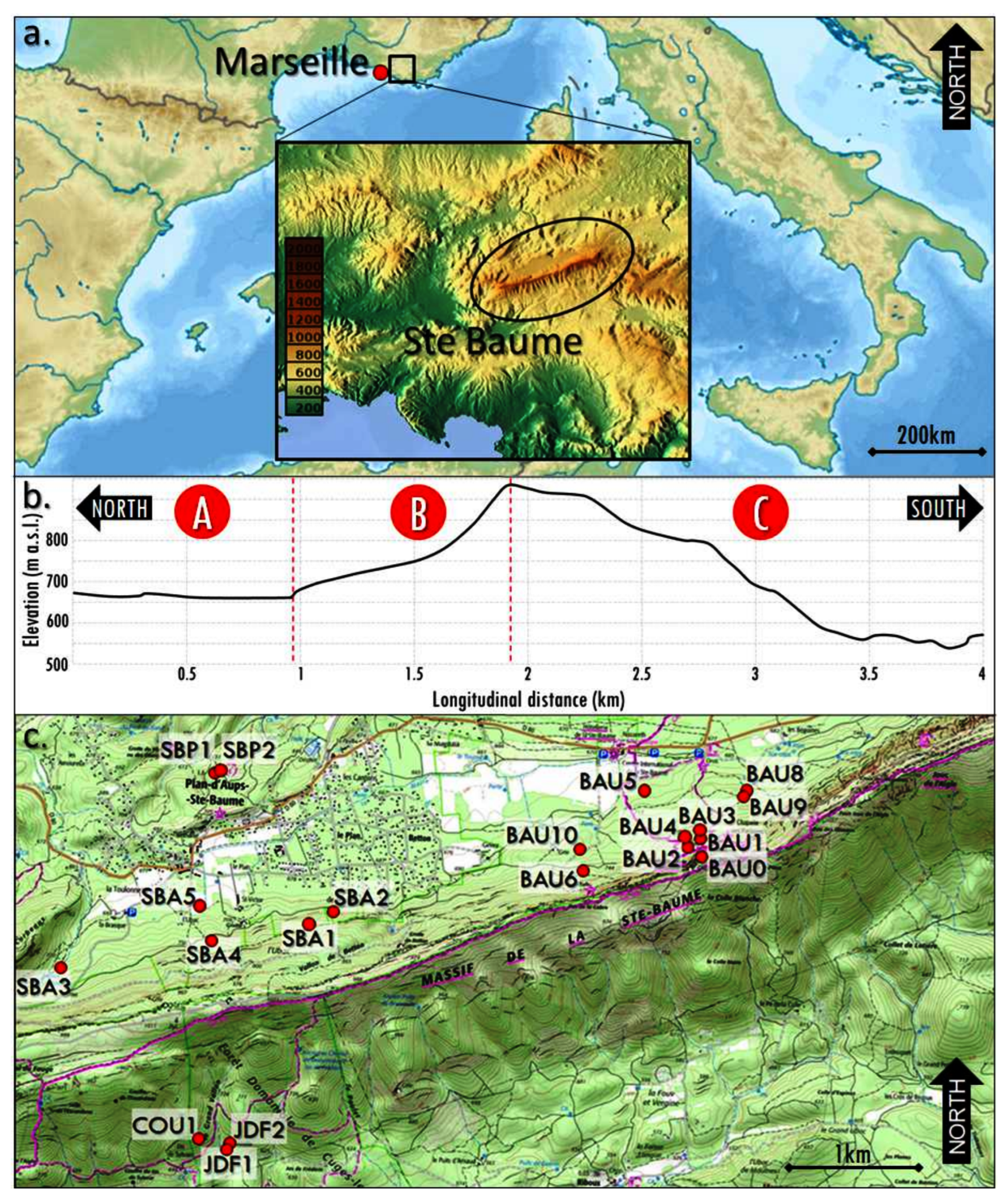
Figure two. Full charcoal concentration by type of archive (all samples cumulated).
Figure 2. Total charcoal concentration past type of archive (all samples cumulated).
Effigy three. Cluster analysis based on Euclidean similarity measures.
Figure 3. Cluster analysis based on Euclidean similarity measures.
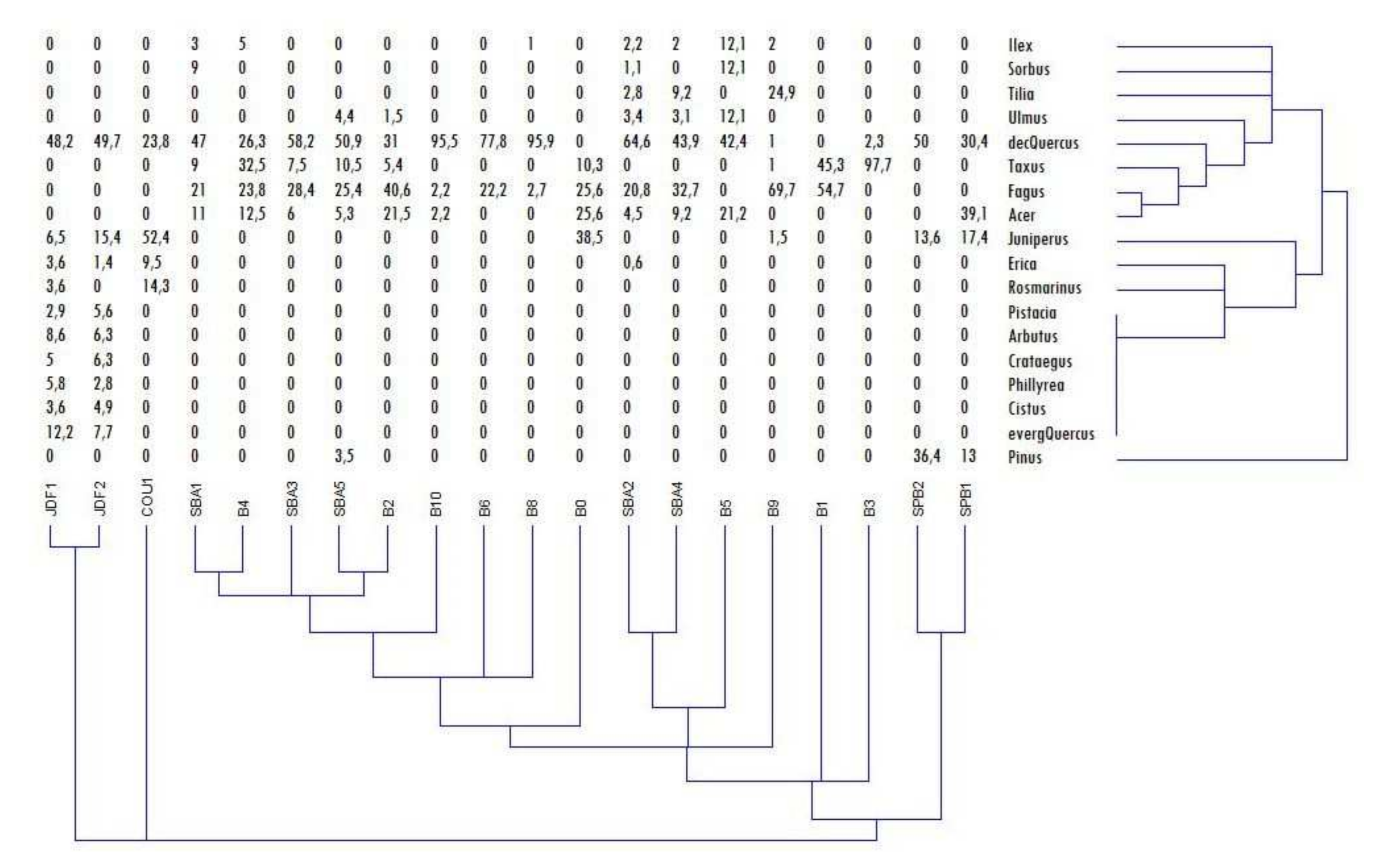
Figure iv. Correspondence analysis of the frequencies of taxa co-ordinate to sampled soil profiles for all 3 ecological units (blueish: southern exposure; black: Sainte-Baume forest; green: northern plateau) related to the successional groups (pioneer: Juniperus and Pinus; scrubland: Ilex, Rosmarinus, Crataegus, Cistus, Pistacia, Phillyrea, evergreen Quercus, Arbutus and Erica; mesophilic: Sorbus, Tilia, Acer, Ulmus and Taxus) and the two about frequent taxa (Fagus and deciduous Quercus).
Effigy 4. Correspondence analysis of the frequencies of taxa according to sampled soil profiles for all three ecological units (blue: southern exposure; black: Sainte-Baume woods; green: northern plateau) related to the successional groups (pioneer: Juniperus and Pinus; scrubland: Ilex, Rosmarinus, Crataegus, Cistus, Pistacia, Phillyrea, evergreen Quercus, Arbutus and Erica; mesophilic: Sorbus, Tilia, Acer, Ulmus and Taxus) and the two almost frequent taxa (Fagus and deciduous Quercus).
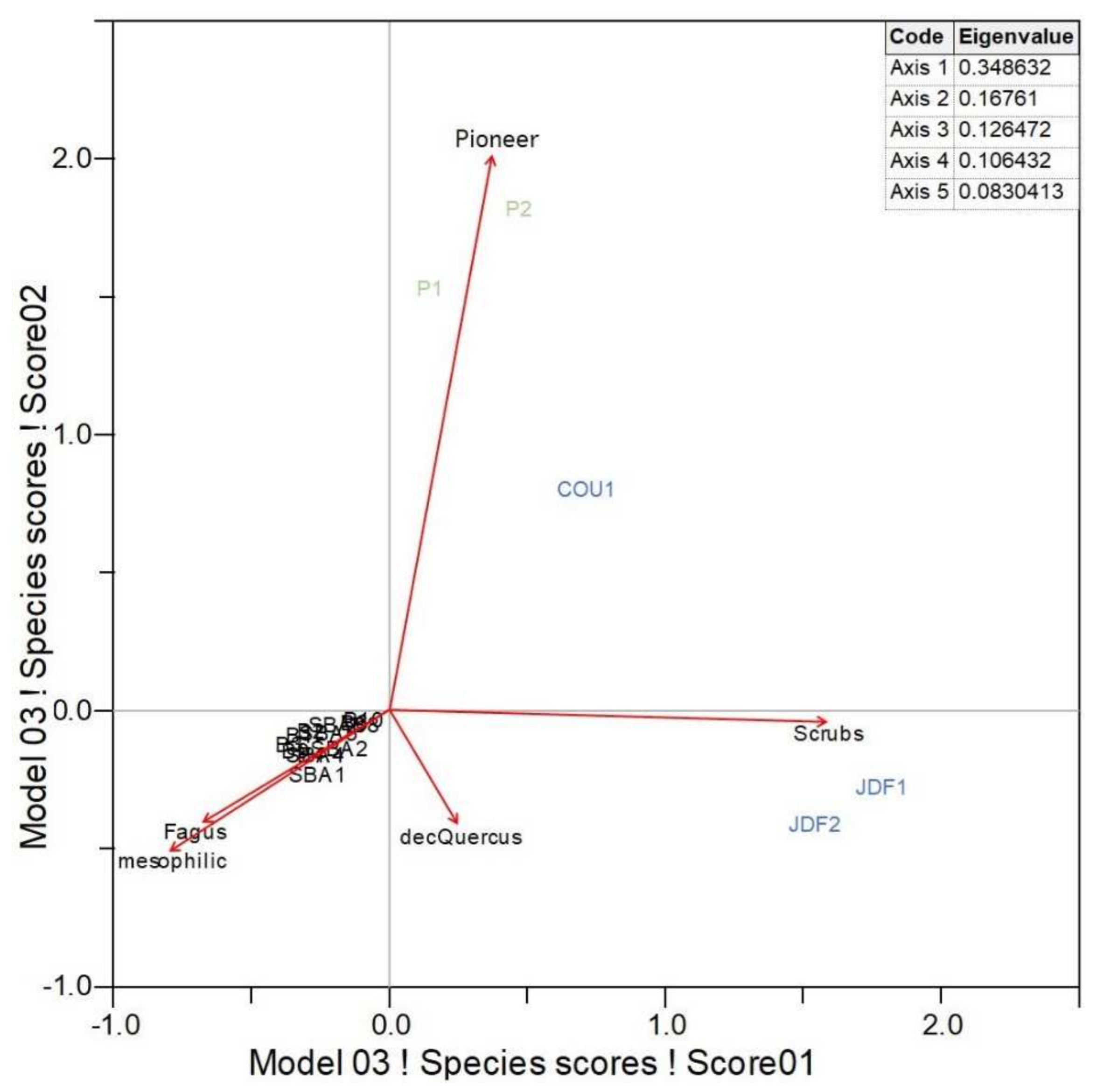
Figure 5. Taxon frequency in the identified soil charcoal assemblages, represented past taxonomic groups with different successional stages (pioneer: Juniperus and Pinus; scrubland: Ilex, Rosmarinus, Crataegus, Cistus, Pistacia, Phillyrea, evergreen Quercus, Arbutus and Erica; mesophilic: Sorbus, Tilia, Acer, Ulmus and Taxus), except that the 2 near frequent taxa are represented separately (Fagus and deciduous Quercus).
Figure v. Taxon frequency in the identified soil charcoal assemblages, represented by taxonomic groups with different successional stages (pioneer: Juniperus and Pinus; scrubland: Ilex, Rosmarinus, Crataegus, Cistus, Pistacia, Phillyrea, evergreen Quercus, Arbutus and Erica; mesophilic: Sorbus, Tilia, Acer, Ulmus and Taxus), except that the two about frequent taxa are represented separately (Fagus and deciduous Quercus).
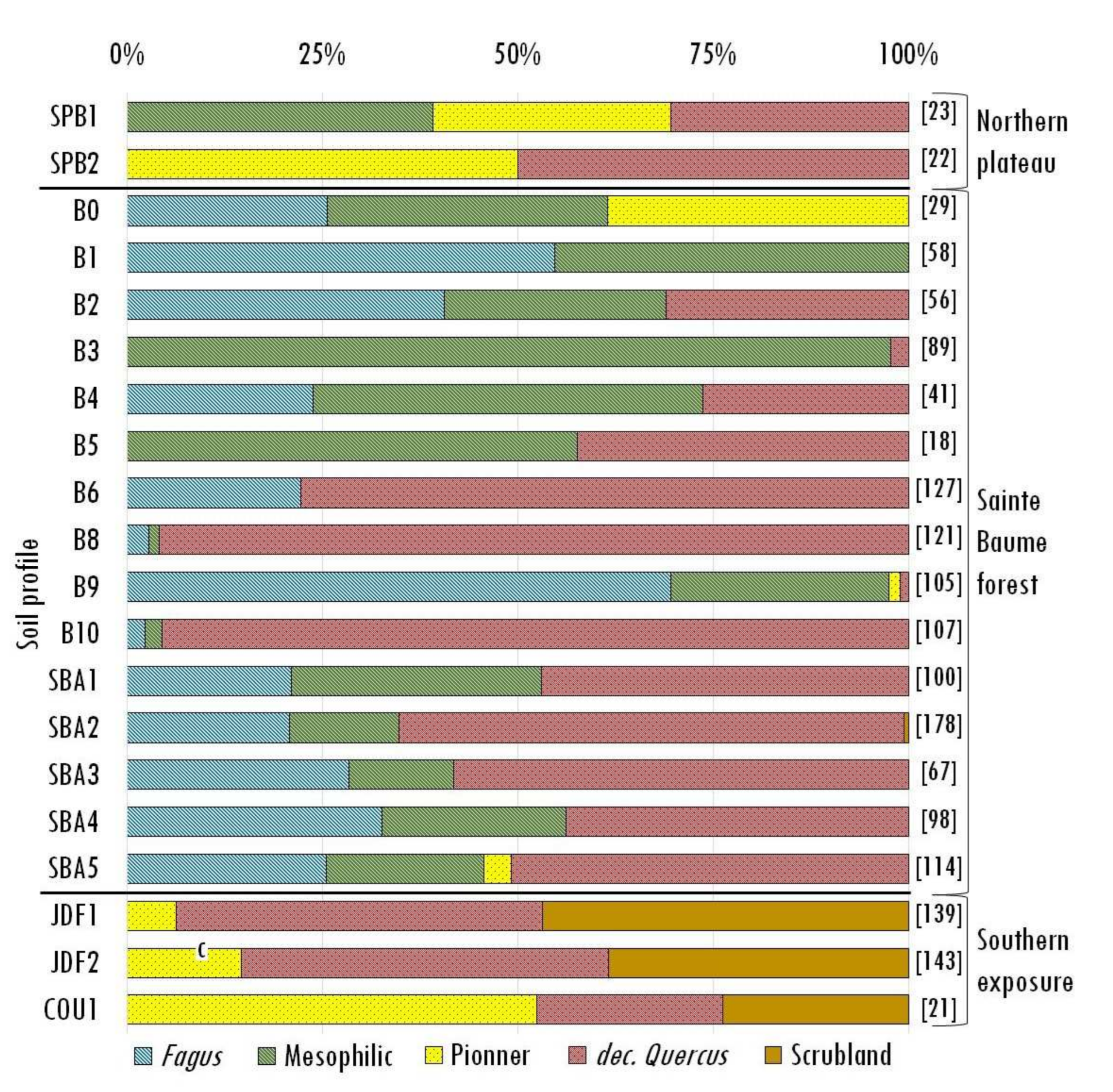
Figure vi. Age distribution of the dated charcoal pieces from the study area for each ecological unit sampled (a) northern plateau; (b) Sainte-Baume forest; and (c) southern exposure) according to dates obtained from 14C accelerated mass spectrometry (AMS) calibrated with a two-standard-deviation (2σ) 95% confidence interval on the OxCal program [60] with the IntCal20 dataset [61].
Figure six. Age distribution of the dated charcoal pieces from the study area for each ecological unit sampled (a) northern plateau; (b) Sainte-Baume forest; and (c) southern exposure) according to dates obtained from 14C accelerated mass spectrometry (AMS) calibrated with a two-standard-divergence (2σ) 95% confidence interval on the OxCal program [60] with the IntCal20 dataset [61].
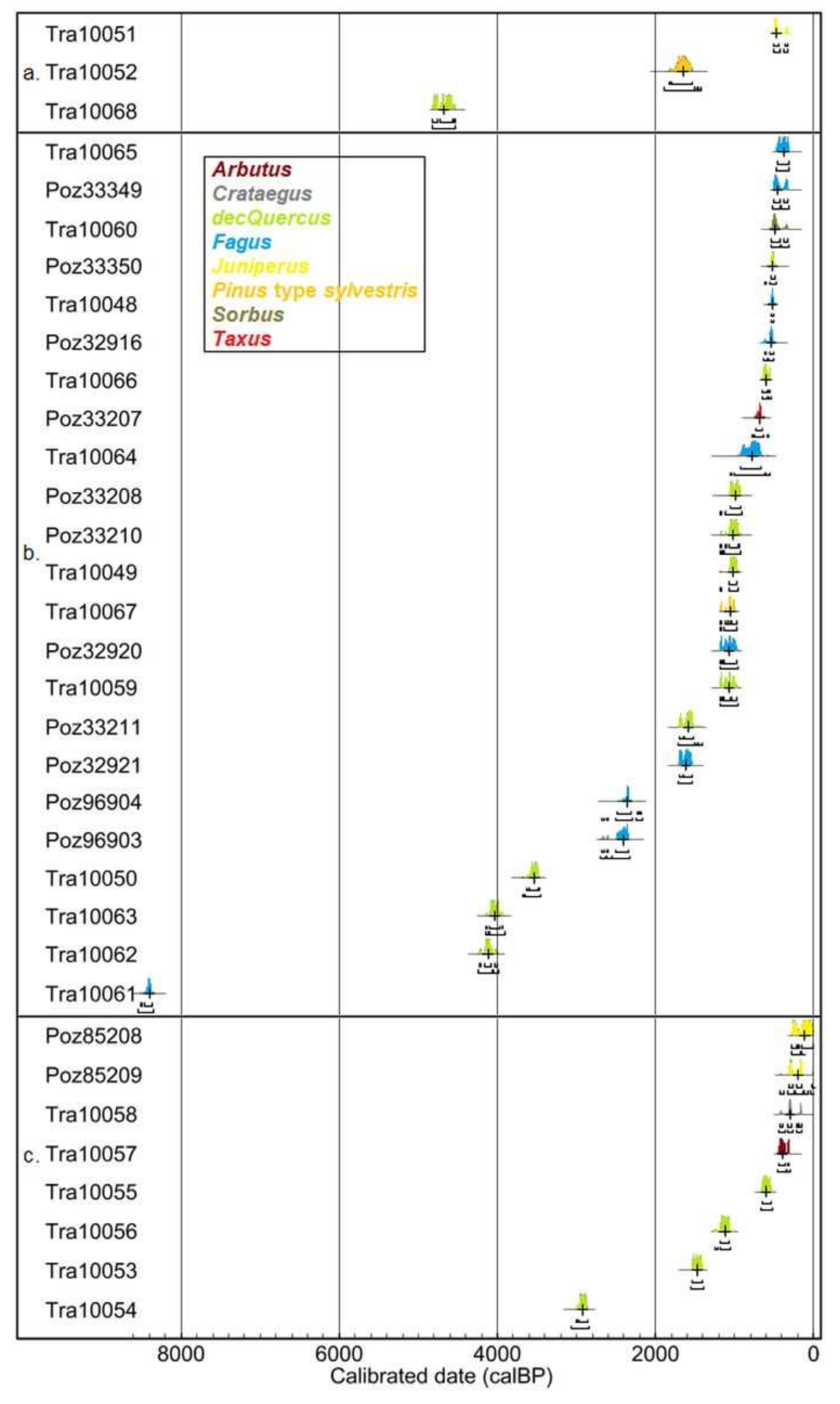
Table one. Overview of the material from sampled soil profiles (* soil classification and soil qualifiers according IUSS Working Group WRB 2015: sk: skeletic, rz: rendzic, co: colluvic, RG: regosols, CM: cambisol).
Table 1. Overview of the textile from sampled soil profiles (* soil classification and soil qualifiers co-ordinate IUSS Working Grouping WRB 2015: sk: skeletic, rz: rendzic, co: colluvic, RG: regosols, CM: cambisol).
| Units of Investigation | Profile | Type of Soil * | Type of Archive | Max Depth (cm) | Nbr Samples | |
|---|---|---|---|---|---|---|
| Coll. | In Situ | |||||
| Northern plateau | SBP1 | sk-rz-RG | x | x | 50 | 3 |
| SBP2 | x | x | 55 | 3 | ||
| Sainte-Baume forest | SBA1 | co-CM | ten | x | 60 | v |
| SBA2 | 10 | x | 70 | 5 | ||
| SBA3 | x | - | 85 | iv | ||
| SBA4 | x | x | 70 | 4 | ||
| SBA5 | x | x | 140 | four | ||
| BAU0 | ten | - | 30 | ii | ||
| BAU1 | x | - | 30 | 2 | ||
| BAU2 | 10 | - | 30 | 2 | ||
| BAU3 | x | - | 30 | 2 | ||
| BAU4 | 10 | - | 30 | ii | ||
| BAU5 | ten | - | 30 | 2 | ||
| BAU6 | ten | - | 50 | 5 | ||
| BAU8 | x | - | fifty | five | ||
| BAU9 | 10 | - | 50 | 5 | ||
| BAU10 | x | - | l | 5 | ||
| Southern exposure | JDF1 | co-rz-RG | x | - | 50 | 5 |
| JDF2 | x | x | 145 | half dozen | ||
| COU1 | sk-rz-RG | x | - | 50 | 2 | |
Tabular array 2. Frequencies of identified taxa in the charcoal assemblages per sampled soil contour.
Table 2. Frequencies of identified taxa in the charcoal assemblages per sampled soil profile.
| Units of Investigation | Profile of Sampling | Nbr of Ident. | Fagus | Juniperus | Acer | Taxus | Dec. Quercus | Everg. Quercus | Ulmus | Ilex | Sorbus | Tilia | Cistus | Pinus | Erica | Phillyrea | Crataegus | Rosmarinus | Arbutus | Pistacia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Northern plateau | SPB1 | 23 | - | 17 | 39 | - | 30 | - | - | - | - | - | - | 13 | - | - | - | - | - | - |
| SPB2 | 22 | - | 14 | - | - | 50 | - | - | - | - | - | - | 36 | - | - | - | - | - | - | |
| Sainte-Baume woods | SBA1 | 100 | 21 | - | 11 | nine | 47 | - | - | iii | 9 | - | - | - | - | - | - | - | - | - |
| SBA2 | 178 | 21 | - | 5 | - | 65 | - | 3 | 2 | ane | iii | - | - | 1 | - | - | - | - | - | |
| SBA3 | 67 | 28 | - | 6 | eight | 58 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| SBA4 | 98 | 33 | - | 9 | - | 44 | - | iii | ii | - | ix | - | - | - | - | - | - | - | - | |
| SBA5 | 114 | 25 | - | 5 | 11 | 51 | - | iv | - | - | - | - | four | - | - | - | - | - | - | |
| B0 | 29 | 26 | 39 | 26 | ten | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| B1 | 58 | 55 | - | - | 45 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| B2 | 56 | 41 | - | 22 | 5 | 31 | - | ii | - | - | - | - | - | - | - | - | - | - | - | |
| B3 | 89 | - | - | - | 98 | 2 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| B4 | 41 | 24 | - | 13 | 33 | 26 | - | - | 5 | - | - | - | - | - | - | - | - | - | - | |
| B5 | 18 | - | - | 21 | - | 42 | - | 12 | 12 | 12 | - | - | - | - | - | - | - | - | - | |
| B6 | 127 | 22 | - | - | - | 78 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| B8 | 121 | 3 | - | - | - | 96 | - | - | 1 | - | - | - | - | - | - | - | - | - | - | |
| B9 | 105 | 70 | two | - | 1 | 1 | - | - | two | - | 25 | - | - | - | - | - | - | - | - | |
| B10 | 107 | 2 | - | 2 | - | 96 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Southern exposure | JDF1 | 139 | - | 7 | - | - | 48 | 12 | - | - | - | - | 4 | - | 4 | 6 | 5 | 4 | 9 | 3 |
| JDF2 | 143 | - | 15 | - | - | 50 | eight | - | - | - | - | 5 | - | 1 | three | 6 | - | 6 | 6 | |
| COU1 | 21 | - | 52 | - | - | 24 | - | - | - | - | - | - | - | 10 | - | - | 14 | - | - |
Tabular array three. Dates of charcoal pieces obtained by 14C accelerator mass spectrometry (AMS) calibrated with a ii-standard-deviation (2σ) 95% confidence interval on the OxCal program [threescore] with the IntCal20 dataset [61].
Table 3. Dates of charcoal pieces obtained by 14C accelerator mass spectrometry (AMS) calibrated with a 2-standard-deviation (2σ) 95% confidence interval on the OxCal programme [sixty] with the IntCal20 dataset [61].
| Units of Investig. | Lab. Ref. | Profile | Taxa | Conv. Historic period BP ± Mistake | Cal. Age BP | Cal. Age BCE/CE |
|---|---|---|---|---|---|---|
| Northern plateau | Tra10051 | SBP1 | Juniperus | 389 ± sixteen | 504-334 | 1446-1616 CE |
| Tra10052 | SBP1 | Pinus type sylvestris | 1770 ± 59 | 1825-1553 | 125-398 CE | |
| Tra10068 | PDA1 | deciduous Quercus | 4129 ± 21 | 4815-4549 | BCE 2866-2600 | |
| Sainte-Baume forest | Tra10060 | SBA1 | Sorbus | 426 ± 40 | 534-325 | 1416-1625 CE |
| Tra10059 | SBA1 | deciduous Quercus | 1162 ± 26 | 1177-987 | 774-963 CE | |
| Tra10062 | SBA1 | deciduous Quercus | 3755 ± 20 | 4224-4000 | BCE 2275-2051 | |
| Tra10063 | SBA1 | deciduous Quercus | 3692 ± 20 | 4090-3973 | BCE 2141-2024 | |
| Tra10061 | SBA1 | Fagus | 7620 ± 31 | 8509-8372 | BCE 6560-6423 | |
| Tra10065 | SBA3 | Fagus | 333 ± xviii | 465-311 | 1486-1639 CE | |
| Tra10066 | SBA3 | deciduous Quercus | 600 ± xv | 646-548 | 1304-1403 CE | |
| Tra10064 | SBA3 | Fagus | 851 ± 85 | 930-666 | 1021-1285 CE | |
| Tra10067 | SBA3 | Pinus type sylvestris | 1155 ± 14 | 1174-987 | 776-963 CE | |
| Tra10048 | SBA4 | Fagus | 494 ± 16 | 537-510 | 1414-1441 CE | |
| Tra10049 | SBA4 | deciduous Quercus | 1126 ± 15 | 1062-979 | 889-971 CE | |
| Tra10050 | SBA4 | deciduous Quercus | 3329 ± 23 | 3632-3480 | BCE 1683-1531 | |
| Poz32916 | BAU1 | Fagus | 525 ± thirty | 629-509 | 1322-1442 CE | |
| Poz33207 | BAU1 | Taxus | 750 ± 35 | 733-658 | 1217-1292 CE | |
| Poz33208 | BAU2 | deciduous Quercus | 1085 ± 35 | 1060-932 | 891-1019 CE | |
| Poz32920 | BAU2 | Fagus | 1160 ± 30 | 1177-983 | 773-968 CE | |
| Poz33210 | BAU3 | deciduous Quercus | 1115 ± 35 | 1173-935 | 778-1015 CE | |
| Poz32921 | BAU6 | Fagus | 1725 ± 30 | 1706-1562 | 245-389 CE | |
| Poz96903 | BAU7 | Fagus | 2385 ± 35 | 2680-2342 | BCE 731-393 | |
| Poz33349 | BAU8 | Fagus | 390 ± 30 | 510-320 | 1441-1631 CE | |
| Poz33350 | BAU8 | deciduous Quercus | 480 ± 30 | 543-498 | 1408-1452 CE | |
| Poz33211 | BAU8 | deciduous Quercus | 1690 ± 35 | 1698-1531 | 253-419 CE | |
| Poz96904 | BAU10 | Fagus | 2340 ± 35 | 2485-2213 | BCE 536-264 | |
| Southern exposure | Tra10058 | JDF1 | Crataegus | 249 ± 26 | 426-0 | 1524-0 CE |
| Tra10057 | JDF1 | Arbutus | 309 ± 14 | 432-306 | 1519-1645 CE | |
| Tra10055 | JDF1 | deciduous Quercus | 599 ± 39 | 655-539 | 1295-1411 CE | |
| Tra10056 | JDF1 | deciduous Quercus | 1208 ± 18 | 1219-1066 | 732-884 CE | |
| Poz85209 | JDF2 | Juniperus | 225 ± thirty | 310-0 | 1640-0 CE | |
| Tra10053 | JDF2 | deciduous Quercus | 1608 ± 23 | 1553-1415 | 397-536 CE | |
| Tra10054 | JDF2 | deciduous Quercus | 2821 ± 21 | 2976-2861 | BCE 1027-912 | |
| Poz85208 | COU1 | Juniperus | 120 ± xxx | 272-eleven | 1679-1940 CE |
| Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Eatables Attribution (CC By) license (https://creativecommons.org/licenses/past/iv.0/).
Source: https://www.mdpi.com/1999-4907/12/11/1541/htm
Posted by: rubioearanting.blogspot.com

0 Response to "The Interactions Of Earthworms And Robins In A Beech Forest Makeup Which Ecological Unit?"
Post a Comment